Environmental Protection Documents

Regulatory Permits
FCC Form 471 Instructions for Schools and Libraries
This file contains the instructions for completing the FCC Form 471, which is essential for schools and libraries to apply for universal service discounts. It provides detailed guidelines on eligibility, filing processes, and compliance requirements. Proper completion of this form is crucial for accessing funding for eligible services.

Regulatory Permits
Underground Storage Tank Notification Form
This form is essential for notifying about underground storage tanks. It helps comply with federal regulations regarding hazardous substances. Owners must complete and submit this form as required by the EPA.

Renewable Energy
Enphase Product Registration Form Instructions
This is a comprehensive product registration form for Enphase products. It contains fields for user contact information, product details, and installation specifics. Completing this form ensures proper registration and updates regarding your Enphase product.

Regulatory Permits
FDA Tobacco Substantial Equivalence Report Submission
This file contains the guidelines and requirements for submitting a Tobacco Substantial Equivalence Report. It is essential for manufacturers and importers seeking marketing authorization for new tobacco products. Follow the outlined instructions to ensure compliance with FDA regulations.
Regulatory Permits
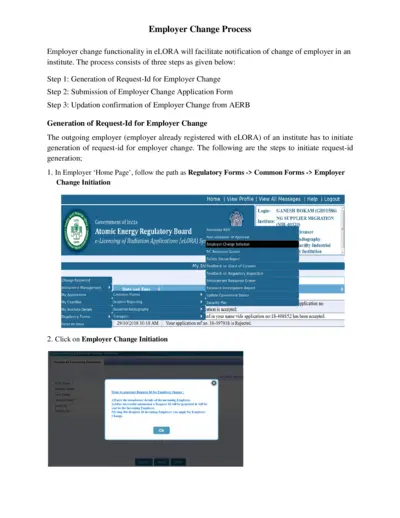
Employer Change Process in eLORA System
This document outlines the employer change process in the eLORA system, guiding users through the steps required for changing employers. It provides a comprehensive view of the procedure, including the generation of request IDs and submission of applications. Ideal for users seeking to navigate the regulatory requirements efficiently.

Wildlife Protection
Ohio Wild Animal Collecting Permits Guide
This document provides essential information on permits related to scientific collecting, education, bird banding, and wildlife rehabilitation in Ohio. It includes necessary qualifications and application processes for individuals involved in wildlife activities. It serves as a summary of the relevant laws and regulations governing such permits.

Regulatory Permits
IRB Application for Greater Than Minimal Risk Research
The IRB Application form is essential for research projects involving greater than minimal risk. This document guides investigators through compliance with research protocols. It includes vital information about the project, investigators, and required approvals.

Renewable Energy
PG&E Medical Baseline Program Self-Certification Request
This file contains essential information and instructions for the PG&E Medical Baseline Program self-certification process. It includes details required for filling out the request, eligibility criteria, and contact information for assistance. Users can benefit from the services and ensure compliance to maintain their eligibility.
Renewable Energy
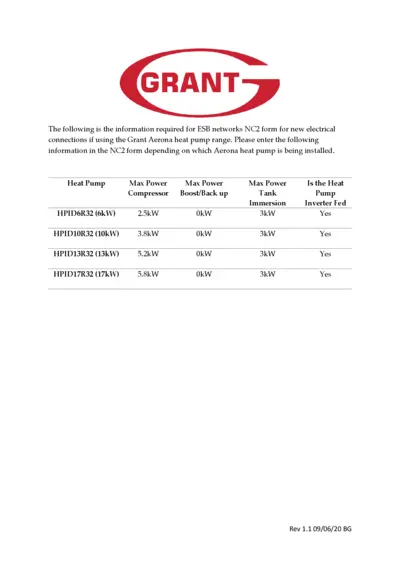
ESB Networks NC2 Form for Grant Aerona Heat Pump
The ESB Networks NC2 form provides essential information for new electrical connections using Grant Aerona heat pumps. This file outlines the specifications and requirements to complete the NC2 form accurately. It's a useful guide for both consumers and business users looking to understand the installation process.

Waste Management
Ramsey County Yard Waste Management Instructions
This file provides essential information about Ramsey County's yard waste management program. Users can find guidelines on how to participate in the program and important milestones related to waste reduction. Ideal for residents looking to manage yard waste responsibly.

Renewable Energy
NYC Energy Code Simplification Proposal
This file outlines proposals to simplify the New York City Energy Code based on ASHRAE 90.1 standards. It addresses compliance, health benefits, and implementation. Aimed at enhancing energy efficiency in commercial and residential buildings.

Regulatory Permits
FDA Form 3938 Drug Master File Instructions
This document provides essential guidelines for completing FDA Form 3938 for Drug Master Files (DMFs). It outlines the necessary steps for various stakeholders involved in the submission process. Use this resource to ensure compliance and streamline your application.