Public Health Documents

Healthcare Policy
Equal Opportunity Sexual Harassment Complaint Process
This file outlines the complaint process for incidents of sexual harassment and discrimination. It provides guidance on informal and formal complaint procedures. Understanding your rights and steps to take can help ensure a supportive environment.

Public Health Research
Human Subjects Consent Form Sample for Research
This file contains a sample Human Subjects Consent Form used for research studies. It outlines key information necessary for potential participants. Understanding this form is essential for ethical participation in research.

Health Insurance Programs
Implementing Health Insurance for the Poor in Karnataka
This file provides insights into the Rashtriya Swasthya Bima Yojana (RSBY) implementation in Karnataka, India. It reports on health insurance access for the poor and the challenges faced with the program rollout. The findings are based on comprehensive surveys and interviews with healthcare providers.
Public Health Research
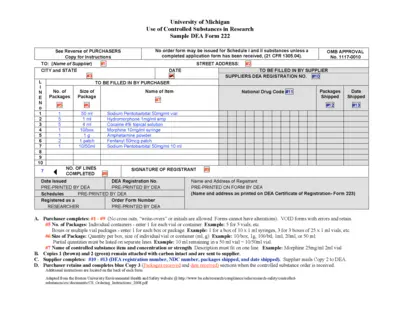
University of Michigan Controlled Substances Form
This file provides a detailed instruction for researchers on how to order controlled substances. It includes essential guidelines for filling out the DEA Form 222. Follow the instructions carefully to ensure compliance with federal regulations.

Public Health Research
Finding Relationship Tendency in Attack on Titan
This research delves into the interpersonal dynamics between Erwin Smith and Levi Ackerman in the manga Attack on Titan. It applies systemic functional linguistics to evaluate their relationship based on their interactions. The study offers insights into fans' interpretations and the complexities of character relationships.

Public Health Research
Instructions for UtahState Research Fund Management
This file contains essential information on managing cash and gift card disbursements for research subjects at Utah State University. It outlines the necessary details, procedures, and responsibilities for researchers and personnel involved. Proper completion of this form ensures compliance and efficient fund management.

Public Health Research
Regulatory Binder Template Section Tabs Overview
This document serves as a comprehensive template for organizing regulatory binders in clinical research. It provides essential instructions and guidelines for maintaining up-to-date regulatory documents. Ideal for investigators and research teams at University Hospitals.

Public Health Research
DBT Junior Research Fellowship for Biotechnology
The DBT-Junior Research Fellowship is open for Indian nationals pursuing research in Biotechnology and Applied Biology. The fellowship supports candidates who have completed or are completing their Masters degree in relevant fields. This opportunity is ideal for those aiming to enroll in a Ph.D. program in India.

Public Health Research
Recording User Input Tool for Human-Computer Interaction
This file introduces the Recording User Input (RUI) tool designed for studying user interactions with software applications. It provides insights into human behavior through detailed timing logs. Ideal for researchers and practitioners in human-computer interaction.

Health Insurance Programs
Guidance on Annual Redeterminations for 2015 Eligibility
This file provides guidance on annual eligibility redeterminations for coverage under the Affordable Care Act for the year 2015. It outlines procedures for the Marketplace regarding eligibility and renewal of health insurance coverage. The document details the responsibilities of both enrollees and the Marketplace in maintaining coverage.

Public Health Research
Visitor Registration Form - National Museum of Natural History
The Visitor Registration Form is essential for individuals wishing to visit the National Museum of Natural History. It collects necessary visitor information and details for research purposes. Completing this form ensures a smooth visit to the museum.

Public Health Research
Piedmont Healthcare Research Services Guide
This document provides essential information and instructions for engaging with the Piedmont Healthcare Office of Research Services. It outlines the roles, responsibilities, and procedures for researchers and affiliated individuals. Utilize this comprehensive guide to ensure compliance and effectiveness in your research endeavors.