Pharmaceuticals Documents

FDA Regulations
FDA Form 1572 Statement of Investigator Guidance
This document serves as a comprehensive guide for the Statement of Investigator (Form FDA 1572). It details the requirements for clinical investigators in research studies. Ideal for sponsors, clinical investigators, and IRBs seeking clarity on FDA guidelines.

FDA Regulations
NDC Labeler Code and Establishment Registration Guide
This document provides essential instructions for generating NDC Labeler Code and Establishment Registration SPL files. It focuses on leveraging FDA's free tools while also mentioning options from vendors for various user needs. Users will find vital resources for submitting SPL files effectively within this guide.

Genetic Engineering
Texas Engineering Practice Act and Firm Registration
This document outlines the Texas Engineering Practice Act and Board rules concerning firm registration. It provides essential FAQs and clear guidelines for both individual and firm registration requirements. Understanding these regulations is crucial for compliance and professional practice in Texas.

Genetic Engineering
Structural Engineer License Application Overview
This file outlines the application process for obtaining a structural engineering license in Washington State, including requirements and submission details. It provides essential information for prospective applicants looking to apply by comity or exam. Follow the outlined steps to ensure a smooth application process.

FDA Regulations
Understanding the Form FDA 483 Process and Timeline
This file presents essential information about the FDA 483 process and its timeline. It is useful for regulatory affairs professionals and companies engaging with the FDA. The guide offers insights into inspection observations and compliance requirements.
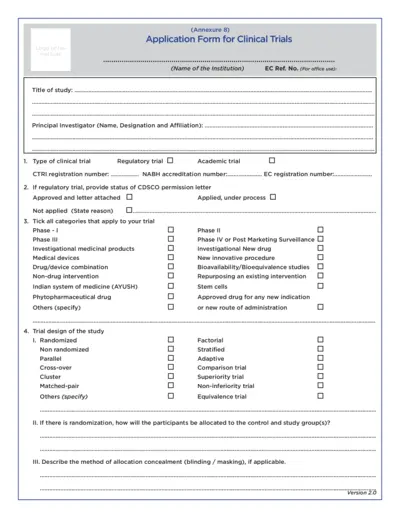
Clinical Trials
Application Form for Clinical Trials
This file is an application form for clinical trials, detailing necessary information for the ethical committee review. It guides researchers in providing essential data regarding their study, interventions, and logistic support. The form ensures that all regulatory and scientific considerations are addressed before initiating clinical trials.

Genetic Engineering
American Seal Application Specification Form
This file contains the application specification form for seals. It provides essential details necessary for seal design and application. Users need to fill out all required fields accurately to ensure proper sealing solutions.

Mail-Order Pharmacies
Commercial Letters and Postcards Standards Guide
This file provides detailed standards and instructions for preparing and mailing commercial letters and postcards using Standard Mail services. It includes eligibility criteria, pricing, and documentation requirements. Ideal for businesses and nonprofit organizations looking to optimize their mailings.