Batch Manufacturing Record for Tongkat Ali Tablet
This document contains the Batch Manufacturing Record for the production of Tongkat Ali 250mg Tablets. It includes essential manufacturing details, production records, and reference documents. Use this file to ensure adherence to production standards and quality management.
Edit, Download, and Sign the Batch Manufacturing Record for Tongkat Ali Tablet
Form
eSign
Add Annotation
Share Form
How do I fill this out?
Filling out this document requires careful attention to detail. Begin by reviewing the essential sections outlined in the Batch Manufacturing Record. Ensure that all information is accurate and fully completed for compliance with production standards.
How to fill out the Batch Manufacturing Record for Tongkat Ali Tablet?
1
Review the previous batch records for reference.
2
Fill in the required fields with accurate data.
3
Ensure all signatures are obtained from responsible personnel.
4
Attach any necessary reference documents as specified.
5
Conduct a final review to confirm completeness before submission.
Who needs the Batch Manufacturing Record for Tongkat Ali Tablet?
1
Quality Assurance teams need this file to ensure compliance with production standards.
2
Production managers require it to oversee the manufacturing process.
3
Regulatory bodies may need it for audits and inspections.
4
New employees will reference this file for training on manufacturing operations.
5
Supply chain managers use it to track material requisition and inventory levels.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the Batch Manufacturing Record for Tongkat Ali Tablet along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your Batch Manufacturing Record for Tongkat Ali Tablet online.
Editing this PDF on PrintFriendly is simple and intuitive. Begin by uploading the PDF document to our platform, where you can make real-time edits. Save your changes directly to your device once you are satisfied with the modifications.
Add your legally-binding signature.
Signing the PDF on PrintFriendly is quick and straightforward. After editing your document, simply navigate to the signature tab to add your signature. Once signed, save the document to keep the changes intact.
Share your form instantly.
Sharing PDFs on PrintFriendly is easy and effective. After you've edited and signed your document, use our share function to distribute it via email or social media. This feature ensures your files reach the intended audience promptly.
How do I edit the Batch Manufacturing Record for Tongkat Ali Tablet online?
Editing this PDF on PrintFriendly is simple and intuitive. Begin by uploading the PDF document to our platform, where you can make real-time edits. Save your changes directly to your device once you are satisfied with the modifications.
1
Upload your PDF document to PrintFriendly.
2
Use the editing tools provided to make necessary changes.
3
Add text, images, or notes as needed for clarity.
4
Preview your edits to ensure accuracy.
5
Save and download the finalized document to your device.

What are the instructions for submitting this form?
To submit this form, please email the completed Batch Manufacturing Record to the quality assurance department at qa@companyname.com. Alternatively, you may fax it to 123-456-7890 or submit it through our internal submission portal. Ensure that all required signatures are obtained prior to submission to avoid any delays.
What are the important dates for this form in 2024 and 2025?
No significant dates apply for the use of this form in 2024 and 2025. It is applicable as long as the records remain current and relevant to production standards.

What is the purpose of this form?
The purpose of this form is to ensure accurate documentation of the manufacturing process for Tongkat Ali Tablets. It serves as a crucial tool for quality assurance, compliance audits, and production management. By documenting each step of the manufacturing process, this form aids in maintaining high standards of product quality.

Tell me about this form and its components and fields line-by-line.

- 1. Product Details: Includes product name, description, and batch quantity.
- 2. Production Batch Record Issuance: Records who issued the batch record and verifies its accuracy.
- 3. Reference Documents: Lists standard operating procedures relevant to the production process.
- 4. Raw Materials: Documents the materials used along with their lot numbers and quantities.
- 5. Processing Equipments: Details on the equipment utilized in the manufacturing process.
- 6. Area Clearance: Confirms the cleanliness and readiness of the production area.
What happens if I fail to submit this form?
Failing to submit this form may lead to delays in production and compliance issues. Without proper documentation, quality assurance processes can be compromised, endangering product integrity.
- Compliance Issues: Insufficient documentation may result in non-compliance with industry regulations.
- Production Delays: Lack of timely submission can halt production processes.
- Quality Assurance Risks: Inaccurate records can lead to quality control problems.
How do I know when to use this form?

- 1. New Batch Production: Documenting details for newly manufactured batches.
- 2. Quality Assurance Audits: Ensuring compliance during internal and external audits.
- 3. Training New Employees: Training resources for new hires in production and management.
Frequently Asked Questions
How do I edit this PDF?
To edit this PDF, upload it to PrintFriendly and use our intuitive editing tools to make your changes.
Can I sign this PDF?
Yes, after editing, you can easily add your signature before saving.
What format can I download this PDF in?
You can download the edited PDF in its original format for your records.
How do I share this document?
Share your PDF via email or social media directly from PrintFriendly.
Is there a limit to how many times I can edit?
No, you can edit the document as many times as you need before you download it.
What if I make a mistake while editing?
You can easily undo changes or re-edit sections of the document.
Can others edit this document?
Yes, you can share the document with others who can then edit it on their accounts.
What if I need to add images?
You can easily insert images into the PDF using the editing tools on PrintFriendly.
Can I revert back to the original document?
Once edited and saved, the original version is not retained on our site.
Is there help available if I need it?
Yes, we provide support and a comprehensive help section to assist you.
Related Documents - Tongkat Ali BMR
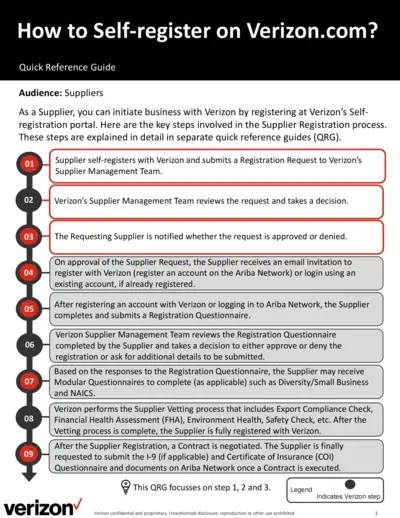
How to Self-register on Verizon.com - Quick Reference Guide
This file provides detailed instructions for suppliers on how to self-register on Verizon.com and submit a registration request. It outlines the steps involved in the supplier registration process, including submitting a registration questionnaire and undergoing a vetting process. The guide also explains how to complete necessary forms and provide required information.

Anonymous Feedback Form for City of Fife Improvements
This form is designed to collect feedback from businesses about possible improvements in housing, land use, transportation, and the environment in the city of Fife. It aims to gather input on how these changes might impact or benefit businesses. The form provides a platform for businesses to share their suggestions for other improvements and includes contact information for further queries.

3M-Matic 800AT Adjustable Case Sealer Instructions
This file contains the instructions and parts list for the 3M-Matic 800AT Adjustable Case Sealer with AccuGlide II Taping Heads. It includes important safety information, spare parts recommendations, and details on machine operation. Consult this manual regularly for proper maintenance to ensure trouble-free operation.
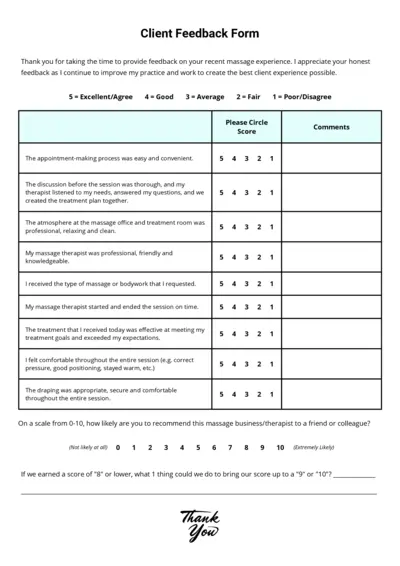
Client Feedback Form for Massage Experience - Easy to Use
This form is designed to gather feedback on your recent massage experience. It helps improve the overall client experience by addressing specific areas of service. Please provide honest feedback to help us serve you better.

SABC Supplier Registration Form
This file is the official form for supplier registration with SABC. It includes sections for company details, director/ownership details, and required documentation. Use this form to apply or update your supplier registration.
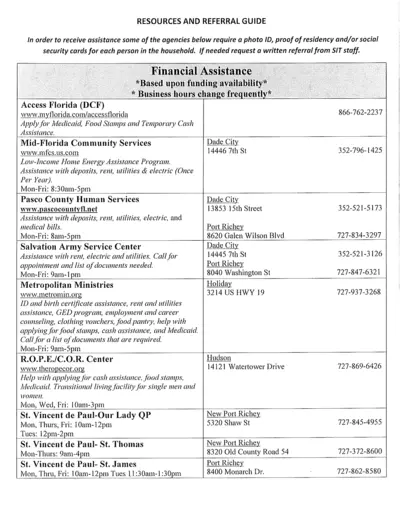
Resources and Referral Guide for Financial Assistance
The Resources and Referral Guide provides detailed information on various agencies offering financial assistance, including requirements and services provided. This guide includes details about programs for rent, utilities, food, clothing, medical bills, and more. Use this guide to find the right support for your needs.

Implement Total Productive Maintenance (TPM) in Manufacturing Industry
This file discusses the implementation of Total Productive Maintenance (TPM) to reduce machine breakdowns and improve productivity. It includes a literature review, case study, and analysis of the effectiveness of TPM. The focus is on autonomous maintenance, planned maintenance, and overall equipment effectiveness.

Ohio Development Reduction in Household Energy Burden
The Ohio Department of Development has allocated funds to reduce energy consumption for income-qualified customers. This document serves as a guideline and grant application instruction for eligible organizations to apply for this program. Funding is available on a first-come, first-serve basis.

Boyfriend Application Form
This Boyfriend Application Form is designed for individuals seeking to evaluate potential partners. It gathers personal information, relationship intentions, personality traits, interests, and lifestyle preferences. The form also includes communication style and other relevant details to help understand and assess compatibility.

Boyfriend Application Form Template
The Boyfriend Application Form Template helps individuals detail their basic information, fun facts, dating history, future aspirations, and compatibility check. It is a comprehensive and engaging way to get to know potential partners better. The template also includes an agreement section.

Part Submission Warrant and Engineering Change Record
This file contains the Part Submission Warrant and related engineering change records for manufacturing organizations. It includes details on part identification, material reporting, and submission results. Essential for complying with Production Part Approval Process requirements.

Marriott Feedback Form - Share Your Thoughts
This file contains the Marriott Feedback Form for customers to share their thoughts. It provides clear instructions on how to submit feedback or complaints. Use this form to ensure your voice is heard effectively.