Edit, Download, and Sign the CHEF Manual Chemical Hazard Engineering Fundamentals
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out this document, begin by reviewing each section thoroughly. Gather the necessary data and follow the prompts provided in the manual. Ensure to double-check your entries before submission.
How to fill out the CHEF Manual Chemical Hazard Engineering Fundamentals?
1
Review the sections of the CHEF Manual carefully.
2
Gather all necessary data and insights.
3
Follow the prompts provided accurately.
4
Double-check each entry for accuracy.
5
Submit the completed form as instructed.
Who needs the CHEF Manual Chemical Hazard Engineering Fundamentals?
1
Chemical engineers who require guided methodologies.
2
Safety professionals looking to improve risk assessment.
3
Managers overseeing chemical processes and safety.
4
Educators teaching chemical engineering concepts.
5
Regulatory bodies seeking standardized practices.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the CHEF Manual Chemical Hazard Engineering Fundamentals along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your CHEF Manual Chemical Hazard Engineering Fundamentals online.
Editing this PDF on PrintFriendly is easy and efficient. Utilize our editor to make necessary changes to your document directly. You can modify, add, or delete content as required.
Add your legally-binding signature.
Signing the PDF on PrintFriendly is simple and convenient. After editing the document, you can add your signature electronically. This feature enhances the ease of completing and finalizing your documents.
Share your form instantly.
Sharing your PDF on PrintFriendly is a breeze. After completing your edits and signing, use the share feature to distribute the document easily. Connect with colleagues or stakeholders effortlessly.
How do I edit the CHEF Manual Chemical Hazard Engineering Fundamentals online?
Editing this PDF on PrintFriendly is easy and efficient. Utilize our editor to make necessary changes to your document directly. You can modify, add, or delete content as required.
1
Upload your PDF to PrintFriendly.
2
Access the editor to make desired changes.
3
Modify text, images, and layout as necessary.
4
Preview the alterations to ensure accuracy.
5
Download the edited document for your records.

What are the instructions for submitting this form?
For submitting the completed form, you can send it via email to submission@chemicalprocesssafety.org. Alternatively, fax it to (555) 123-4567. Physical submissions can be sent to our headquarters located at 123 Safety Lane, Chemical City, CA 90210.
What are the important dates for this form in 2024 and 2025?
Important dates for this form in 2024 and 2025 include annual compliance reviews and updates to safety protocols. Ensure to check periodically for any amendments or new revisions.

What is the purpose of this form?
The purpose of the CHEF Manual is to provide guidance on chemical hazard engineering. It outlines methods for risk analysis and promotes safety standards in the industry. This manual equips professionals with the knowledge to conduct effective risk assessments.

Tell me about this form and its components and fields line-by-line.

- 1. Title: The title of the document for identification.
- 2. Version: The version number for tracking updates.
- 3. Revision Date: The date of the latest revision to ensure current information.
- 4. Document Owner: The individual responsible for maintaining the document.
What happens if I fail to submit this form?
Failure to submit this form could result in delayed risk assessments and potential safety oversights. It's crucial to complete and submit the document to adhere to safety protocols.
- Safety Risks: Inadequate risk assessment increases the chance of chemical incidents.
- Regulatory Non-compliance: Failure to submit could lead to violations and penalties.
- Data Inaccuracies: Incomplete forms may result in incorrect hazard evaluations.
How do I know when to use this form?

- 1. Risk Analysis: Utilize this form during the evaluation of potential chemical hazards.
- 2. Regulatory Documentation: Complete this form to meet compliance requirements.
- 3. Safety Training: Use the insights from this form in training sessions.
Frequently Asked Questions
How do I edit this PDF?
Upload the PDF to PrintFriendly and use the editing tools to modify text and images.
Can I sign the PDF electronically?
Yes, you can add an electronic signature after editing your PDF.
How do I share the PDF after editing?
Use the share feature to send your edited PDF via email or link.
Is there a limit to how many times I can edit a PDF?
You can edit your PDF as many times as you need before downloading.
Can I download the edited PDF?
Absolutely! After making your edits, you can download the updated document.
Are there templates available for this PDF?
While specific templates aren't available, the editing tools are flexible for your needs.
How do I ensure my changes are saved?
Your edits are updated in real-time; simply download once you finish altering the document.
What if I have trouble editing my PDF?
You can reach our support for assistance with any editing issues.
Can I access my edited PDFs later?
Currently, you’ll need to download them immediately after editing.
What file formats can I upload?
You can upload PDF files directly for editing.
Related Documents - CHEF Manual

FDA Recall Audit Check Report Instructions
This file provides detailed instructions for completing the FDA Recall Audit Check Report. It includes information on recall details, program data, audit accounts, and consignee data. Useful for those involved in managing FDA recalls.

Assessment of Abuse Potential of Drugs Guidance for Industry
This document provides guidance for the assessment of abuse potential in drugs. It covers key decision points, recommended studies, and the process for NDA submission. This is crucial for ensuring drug safety and regulatory compliance.

Nurtec ODT Savings Program Terms & Conditions
This document provides detailed terms and conditions for the Nurtec ODT Savings Program. It includes eligibility criteria, instructions for pharmacists, and important disclaimers. Patients using the copay card should adhere to these guidelines to benefit from the program.
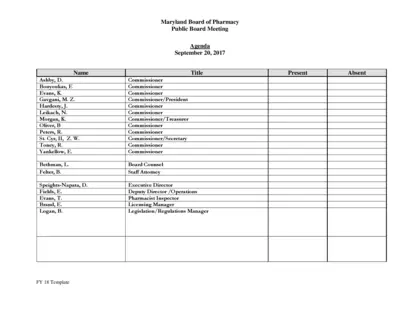
Maryland Board of Pharmacy Public Board Meeting Agenda
This file contains the agenda for the Maryland Board of Pharmacy's public board meeting on September 20, 2017. It includes reports from various committees and updates on operations, licensing, compliance, and more. The document is essential for stakeholders to keep track of board activities and decisions.

Abbreviations for Pharma Manufacturers
This file contains a list of manufacturers' abbreviations organized alphabetically, helping users to identify manufacturer names and their corresponding abbreviations.

Pharma-Lagom: Safe and Effective Medication Use
Pharma-Lagom is a comprehensive guide on the risks and benefits of medication use, aimed at promoting safe and effective medication practices. It includes contributions from experts in the Pharmacy Department of Kalaniketan Polytechnic College, Jabalpur. This document also covers recent events and achievements within the department.

MDUFMA User Fees Cover Sheet Instructions
The MDUFMA User Fees Cover Sheet is required for Medical Device Application Submission. It includes details on registration and payment processes. Follow this guide to complete and submit your form correctly.
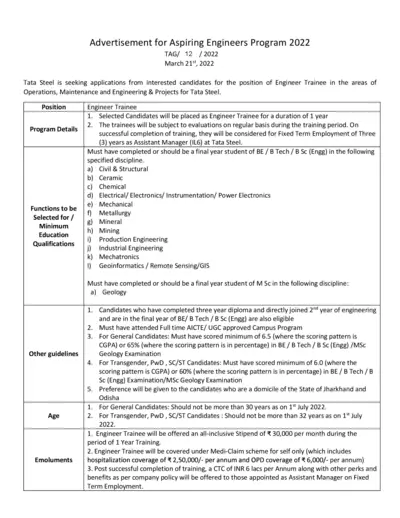
Tata Steel Aspiring Engineers Program 2022 Application
This file includes details about Tata Steel's Aspiring Engineers Program 2022. It covers program details, eligibility criteria, and the application process. It also provides information on evaluation, guidelines, and submission deadlines.

Welding Procedure Specification (WPS) PDF Guide
This file is a Welding Procedure Specification (WPS) that provides detailed instructions for welding procedures, joint design, base metals, filler metals, and more. It includes sections for prequalified and qualified-by-testing procedures. This document is essential for ensuring weld quality and consistency.

California Intern Pharmacist Application Instructions
This document provides detailed instructions for applying for an Intern Pharmacist license in California. It covers processing time, required materials, and special cases for expedited review. Ensure all requirements are met to avoid application delays.

Botox Cosmetic Patient Medication Information
This file contains detailed information about Botox Cosmetic (onabotulinumtoxinA). It includes dosage, administration, warnings, precautions, and adverse reactions. The document is intended for healthcare professionals and patients.

Join the Kings Club and Save Instantly with a Kings Club Card
Apply for a Kings Club Card at any of our locations and start saving instantly. Fill out the form in-store or online to receive your card. Enjoy discounts and additional benefits with your Kings Club membership.