Clinical Trial Application Form Overview
This file provides a comprehensive clinical trial application form for requesting authorization from competent authorities. It includes critical sections for clinical trials on medicinal products for human use. Designed for sponsors, it guides users through necessary information for submission.
Edit, Download, and Sign the Clinical Trial Application Form Overview
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out this form, first gather all necessary information required for the application. Next, ensure that the sections are filled accurately according to guidelines provided. Finally, double-check for completeness and clarity before submission.
How to fill out the Clinical Trial Application Form Overview?
1
Collect relevant information about the clinical trial.
2
Fill in the details of the sponsor and trial.
3
Ensure all sections are completed as per the guidelines.
4
Review the application for any missing information.
5
Submit the completed form to the relevant authority.
Who needs the Clinical Trial Application Form Overview?
1
Clinical researchers who require authorization for trials.
2
Pharmaceutical companies needing to submit trials for review.
3
Ethics committees assessing proposed clinical trials.
4
Regulatory bodies reviewing clinical trial submissions.
5
Sponsors of clinical trials responsible for documentation.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the Clinical Trial Application Form Overview along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your Clinical Trial Application Form Overview online.
Editing this PDF on PrintFriendly is simple and intuitive. You can annotate, highlight, or modify the text as needed for clarity. Our tools make it easy to ensure your document is accurate and professional.
Add your legally-binding signature.
Signing this PDF is streamlined on PrintFriendly. Users can add their signature electronically, ensuring the document is ready for submission. This efficient signing process saves time while maintaining the formality required.
Share your form instantly.
Our platform allows easy sharing of your PDF. You can share your signed or edited documents directly via email or social media. This feature enhances collaboration and accessibility for all users.
How do I edit the Clinical Trial Application Form Overview online?
Editing this PDF on PrintFriendly is simple and intuitive. You can annotate, highlight, or modify the text as needed for clarity. Our tools make it easy to ensure your document is accurate and professional.
1
Open the PDF file in PrintFriendly's editing tool.
2
Select the area you want to modify and make your changes.
3
Use annotations to add comments or highlights as needed.
4
Preview your edits to ensure everything is correct.
5
Save or download your edited PDF directly from the platform.

What are the instructions for submitting this form?
To submit this form, ensure that all fields are filled out accurately and completely. Email the completed form to the designated competent authority, or submit it through the online portal provided. For further instruction, contact our support team or refer to the relevant regulatory guidelines available on our website.
What are the important dates for this form in 2024 and 2025?
Important dates related to this form include submission deadlines for clinical trial applications and expected response times from regulatory bodies. In 2024, keep an eye out for updated guidelines and potential changes in submission requirements. Check back regularly for updates to stay compliant with all legal obligations.

What is the purpose of this form?
The primary purpose of this form is to facilitate the application process for clinical trials involving medicinal products for human use. It serves as a comprehensive guide for sponsors to provide necessary details, ensuring compliance with regulatory standards. Ultimately, it streamlines the authorization process by ensuring all relevant information is presented clearly and accurately.

Tell me about this form and its components and fields line-by-line.

- 1. A.1 - Member State: Indicates the country in which the trial is being conducted.
- 2. A.2 - EudraCT number: A unique identifier assigned to the clinical trial.
- 3. A.3 - Full title of the trial: The complete title as it appears in official documentation.
- 4. B.1 - Sponsor details: Information regarding the organization or individual responsible for the trial.
- 5. B.2 - Legal representatives: Details on any legal representatives acting on behalf of the sponsor.
What happens if I fail to submit this form?
Failing to submit this form can result in delays or denials in authorizing a clinical trial. It's crucial to ensure that all required fields are accurately filled to avoid such issues. Missing documentation may also lead to the need for resubmissions or extended review timelines.
- Delay in Trial Approval: Incomplete or improperly filled forms can lead to significant delays in the trial approval process.
- Rejection of Application: If key information is missing, the competent authority may reject the application outright.
- Increased Costs: Delays in approval could increase operational costs related to the trial.
How do I know when to use this form?

- 1. Regulatory Approval: Essential for obtaining initial approval to conduct clinical trials.
- 2. Ethics Committee Review: Used to present for review by ethics committees.
- 3. Funding Applications: Necessary when seeking funding or investment for the clinical trial.
Frequently Asked Questions
Can I edit the PDF after downloading?
Yes, you can edit the PDF using our tools before downloading it.
Is there a limit to the number of edits?
No, you can make as many edits as necessary before finalizing your document.
Can multiple users collaborate on this form?
Absolutely, you can share the PDF with others for collaborative editing.
What formats can I download the PDF in?
You can download the edited PDF in standard formats suitable for print or online submission.
Will my changes be saved automatically?
Changes need to be saved manually, but the platform allows for multiple save points during editing.
Can I print the PDF directly from PrintFriendly?
Yes, you can print your document directly once you've completed your edits.
Is signing secure on PrintFriendly?
Yes, the signing process is verified and ensures your signature is secure.
How do I share the PDF with others?
You can share your PDF via a share link or by directly sending it through email.
Can I revert to a previous version of the PDF?
Printing or saving previous versions is recommended as a backup before final edits.
What if I encounter issues while editing?
Our support team is available to assist you with any editing issues you may encounter.
Related Documents - CTA Form

FDA Recall Audit Check Report Instructions
This file provides detailed instructions for completing the FDA Recall Audit Check Report. It includes information on recall details, program data, audit accounts, and consignee data. Useful for those involved in managing FDA recalls.

Assessment of Abuse Potential of Drugs Guidance for Industry
This document provides guidance for the assessment of abuse potential in drugs. It covers key decision points, recommended studies, and the process for NDA submission. This is crucial for ensuring drug safety and regulatory compliance.

Nurtec ODT Savings Program Terms & Conditions
This document provides detailed terms and conditions for the Nurtec ODT Savings Program. It includes eligibility criteria, instructions for pharmacists, and important disclaimers. Patients using the copay card should adhere to these guidelines to benefit from the program.
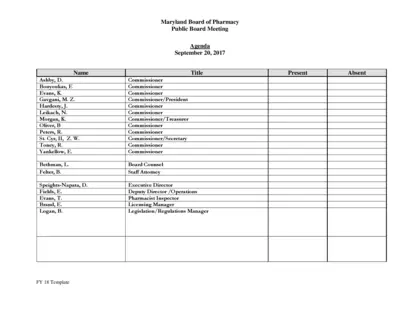
Maryland Board of Pharmacy Public Board Meeting Agenda
This file contains the agenda for the Maryland Board of Pharmacy's public board meeting on September 20, 2017. It includes reports from various committees and updates on operations, licensing, compliance, and more. The document is essential for stakeholders to keep track of board activities and decisions.

Abbreviations for Pharma Manufacturers
This file contains a list of manufacturers' abbreviations organized alphabetically, helping users to identify manufacturer names and their corresponding abbreviations.

Pharma-Lagom: Safe and Effective Medication Use
Pharma-Lagom is a comprehensive guide on the risks and benefits of medication use, aimed at promoting safe and effective medication practices. It includes contributions from experts in the Pharmacy Department of Kalaniketan Polytechnic College, Jabalpur. This document also covers recent events and achievements within the department.

MDUFMA User Fees Cover Sheet Instructions
The MDUFMA User Fees Cover Sheet is required for Medical Device Application Submission. It includes details on registration and payment processes. Follow this guide to complete and submit your form correctly.
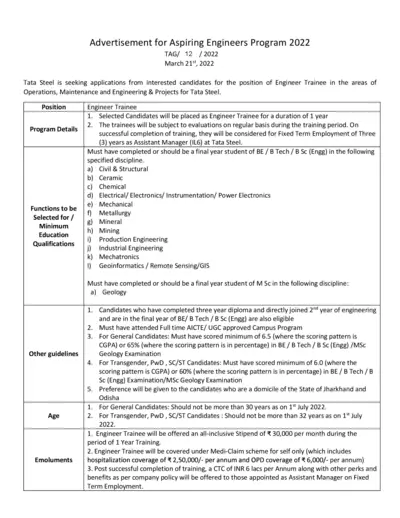
Tata Steel Aspiring Engineers Program 2022 Application
This file includes details about Tata Steel's Aspiring Engineers Program 2022. It covers program details, eligibility criteria, and the application process. It also provides information on evaluation, guidelines, and submission deadlines.

Welding Procedure Specification (WPS) PDF Guide
This file is a Welding Procedure Specification (WPS) that provides detailed instructions for welding procedures, joint design, base metals, filler metals, and more. It includes sections for prequalified and qualified-by-testing procedures. This document is essential for ensuring weld quality and consistency.

California Intern Pharmacist Application Instructions
This document provides detailed instructions for applying for an Intern Pharmacist license in California. It covers processing time, required materials, and special cases for expedited review. Ensure all requirements are met to avoid application delays.

Botox Cosmetic Patient Medication Information
This file contains detailed information about Botox Cosmetic (onabotulinumtoxinA). It includes dosage, administration, warnings, precautions, and adverse reactions. The document is intended for healthcare professionals and patients.

Join the Kings Club and Save Instantly with a Kings Club Card
Apply for a Kings Club Card at any of our locations and start saving instantly. Fill out the form in-store or online to receive your card. Enjoy discounts and additional benefits with your Kings Club membership.