CTO Clinical Trial Centre Initial Application Form
This document provides essential information and guidelines for submitting the Clinical Trial Centre Initial Application Form for research ethics approvals. It outlines revisions, updates, and crucial components for successfully filling out the form. Researchers and coordinators will find valuable instructions on compliance and submission processes.
Edit, Download, and Sign the CTO Clinical Trial Centre Initial Application Form
Form
eSign
Add Annotation
Share Form
How do I fill this out?
Filling out this form requires attention to detail and compliance with guidelines provided. Ensure that all mandatory fields are completed accurately. This section will guide you through the essential steps for proper submission.
How to fill out the CTO Clinical Trial Centre Initial Application Form?
1
Read the guidelines thoroughly before starting your application.
2
Fill out all mandatory fields accurately and completely.
3
Provide any required documentation as specified in the instructions.
4
Double-check your entries for accuracy and completeness.
5
Submit the form following the instructions provided later in this document.
Who needs the CTO Clinical Trial Centre Initial Application Form?
1
Researchers who are initiating clinical trials need this form for ethical approvals.
2
Clinical trial coordinators require it to submit applications to the ethics board.
3
Institutional representatives need to review and sign off on the document.
4
Regulatory bodies use the submitted form for compliance monitoring.
5
Data management teams should have it for tracking consent and participant information.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the CTO Clinical Trial Centre Initial Application Form along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your CTO Clinical Trial Centre Initial Application Form online.
Editing this PDF on PrintFriendly is straightforward and user-friendly. You can click on any field to add or modify the information as needed. Once you are satisfied with the changes, simply download the updated document.
Add your legally-binding signature.
Signing the PDF on PrintFriendly is easy and efficient. You can utilize the built-in signature feature to add your signature electronically. After signing, ensure you save the document for your records.
Share your form instantly.
Sharing the PDF on PrintFriendly is a simple process. You can generate a shareable link that others can use to access the document. This feature makes collaboration more effective and efficient.
How do I edit the CTO Clinical Trial Centre Initial Application Form online?
Editing this PDF on PrintFriendly is straightforward and user-friendly. You can click on any field to add or modify the information as needed. Once you are satisfied with the changes, simply download the updated document.
1
Open the PDF file on PrintFriendly's platform.
2
Click on the fields you want to edit and enter your information.
3
Add any additional notes or comments as needed.
4
Once finished, review your edits for accuracy.
5
Download the edited document or share it directly from the platform.

What are the instructions for submitting this form?
To submit the CTO Clinical Trial Centre Initial Application Form, please send the completed document to the designated ethics board email: ethicsboard@example.com. Alternatively, you can fax it to (123) 456-7890 or submit it through our online submission portal. For physical submissions, please send it to our office at 123 Research Lane, City, State, 45678. Ensure all required fields are completed and necessary documents are attached with your application.
What are the important dates for this form in 2024 and 2025?
Important dates for the submission of this form in 2024 and 2025 will include deadlines set by the ethics approval process. Ensure you are aware of any timelines communicated by the Research Ethics Board or CTO. Check the guidelines regularly for updates on submission deadlines.

What is the purpose of this form?
The purpose of the CTO Clinical Trial Centre Initial Application Form is to ensure that all clinical trials conducted are ethical and compliant with existing regulations. This form facilitates the approval process by providing a structured framework for researchers to submit necessary information and documentation. By streamlining the application process, this form aims to enhance the quality of clinical research and participant safety.

Tell me about this form and its components and fields line-by-line.

- 1. Study Title: The complete title of the study as per the protocol.
- 2. Study ID/Number: Additional identifier for tracking the study.
- 3. Acronym/Short Title: A short title or acronym to identify the research.
- 4. Principal Investigator Details: Contact information for the lead investigator.
- 5. Centre Information: Details regarding the clinical trial center conducting the research.
What happens if I fail to submit this form?
If the application form is not submitted properly, it may result in delays in the ethics approval process. Missing information or documentation can lead to requests for resubmission, which prolongs the timeline for starting the trial. It is crucial to review the application thoroughly before submission.
- Incomplete Information: Failure to provide necessary information may result in form rejection.
- Missing Signatures: Important signatures must be included to validate the application.
- Incorrect Formatting: The form must adhere to specified formatting guidelines; otherwise, it will be returned.
- Late Submission: Submitting after the deadline can negatively impact approval timelines.
- Lack of Supporting Documents: Not including required documents may lead to inquiries and delays.
How do I know when to use this form?

- 1. New Clinical Trial: Use this form to apply for approval to start a new trial.
- 2. Resubmission After Feedback: Utilize it to address comments or changes requested from previous submissions.
- 3. Revision of Existing Studies: Necessary for modifying ongoing trials based on new data.
- 4. Response to Regulatory Changes: Must be completed when regulations affecting the study alter its protocol.
- 5. Addendum for Participant Safety: Required if any changes affect the well-being of study participants.
Frequently Asked Questions
How do I download the completed PDF?
After editing, simply click the download button to save your completed PDF.
Can I edit my PDF on PrintFriendly?
Yes, you can easily edit your PDF using PrintFriendly's user-friendly tools.
Is there a limit to the number of PDFs I can edit?
There is no limit; you can edit as many PDFs as you need.
What if I make a mistake while editing?
You can always go back and make changes until you're satisfied with your document.
Can I print the PDF after editing?
Absolutely! After making your edits, you can print the document directly.
How do I share my edited PDF?
You can generate a link to share your edited PDF with others.
Do I need an account to edit and download PDFs?
No, you can edit and download PDFs without creating an account.
Can I use PrintFriendly on mobile devices?
Yes, PrintFriendly is optimized for use on mobile devices as well.
Is there a cost to use PrintFriendly?
PrintFriendly is free to use for editing and downloading PDFs.
What formats can I download my PDF in?
You can download your PDF in the standard PDF format.
Related Documents - CTO Application Form

FDA Recall Audit Check Report Instructions
This file provides detailed instructions for completing the FDA Recall Audit Check Report. It includes information on recall details, program data, audit accounts, and consignee data. Useful for those involved in managing FDA recalls.

Assessment of Abuse Potential of Drugs Guidance for Industry
This document provides guidance for the assessment of abuse potential in drugs. It covers key decision points, recommended studies, and the process for NDA submission. This is crucial for ensuring drug safety and regulatory compliance.

Nurtec ODT Savings Program Terms & Conditions
This document provides detailed terms and conditions for the Nurtec ODT Savings Program. It includes eligibility criteria, instructions for pharmacists, and important disclaimers. Patients using the copay card should adhere to these guidelines to benefit from the program.
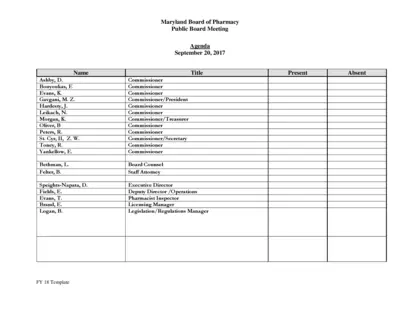
Maryland Board of Pharmacy Public Board Meeting Agenda
This file contains the agenda for the Maryland Board of Pharmacy's public board meeting on September 20, 2017. It includes reports from various committees and updates on operations, licensing, compliance, and more. The document is essential for stakeholders to keep track of board activities and decisions.

Abbreviations for Pharma Manufacturers
This file contains a list of manufacturers' abbreviations organized alphabetically, helping users to identify manufacturer names and their corresponding abbreviations.

Pharma-Lagom: Safe and Effective Medication Use
Pharma-Lagom is a comprehensive guide on the risks and benefits of medication use, aimed at promoting safe and effective medication practices. It includes contributions from experts in the Pharmacy Department of Kalaniketan Polytechnic College, Jabalpur. This document also covers recent events and achievements within the department.

MDUFMA User Fees Cover Sheet Instructions
The MDUFMA User Fees Cover Sheet is required for Medical Device Application Submission. It includes details on registration and payment processes. Follow this guide to complete and submit your form correctly.
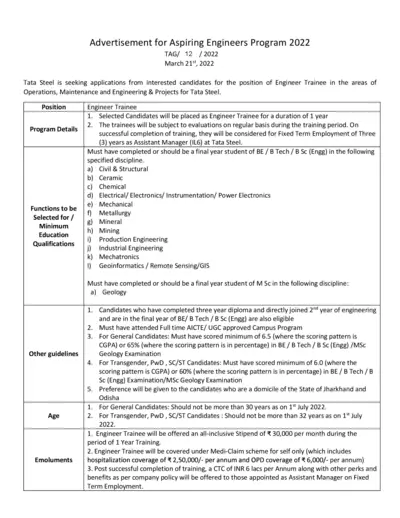
Tata Steel Aspiring Engineers Program 2022 Application
This file includes details about Tata Steel's Aspiring Engineers Program 2022. It covers program details, eligibility criteria, and the application process. It also provides information on evaluation, guidelines, and submission deadlines.

Welding Procedure Specification (WPS) PDF Guide
This file is a Welding Procedure Specification (WPS) that provides detailed instructions for welding procedures, joint design, base metals, filler metals, and more. It includes sections for prequalified and qualified-by-testing procedures. This document is essential for ensuring weld quality and consistency.

California Intern Pharmacist Application Instructions
This document provides detailed instructions for applying for an Intern Pharmacist license in California. It covers processing time, required materials, and special cases for expedited review. Ensure all requirements are met to avoid application delays.

Botox Cosmetic Patient Medication Information
This file contains detailed information about Botox Cosmetic (onabotulinumtoxinA). It includes dosage, administration, warnings, precautions, and adverse reactions. The document is intended for healthcare professionals and patients.

Join the Kings Club and Save Instantly with a Kings Club Card
Apply for a Kings Club Card at any of our locations and start saving instantly. Fill out the form in-store or online to receive your card. Enjoy discounts and additional benefits with your Kings Club membership.