Forms FDA 3542a and 3542 Overview and Instructions
This file provides essential information regarding Forms FDA 3542a and 3542, including their purpose and instructions for use. It is vital for individuals submitting patent information to the FDA. Detailed guidance on how to fill out these forms is also included.
Edit, Download, and Sign the Forms FDA 3542a and 3542 Overview and Instructions
Form
eSign
Add Annotation
Share Form
How do I fill this out?
Filling out Forms FDA 3542a and 3542 involves providing accurate patent and application details. Ensure all required information is completed to avoid submission delays. Follow the specific instructions outlined in the subsequent sections for detailed guidance.
How to fill out the Forms FDA 3542a and 3542 Overview and Instructions?
1
Review the form details and required information.
2
Complete all sections of the form accurately.
3
Attach any necessary supporting documents.
4
Double-check the information for accuracy.
5
Submit the completed form to the FDA accordingly.
Who needs the Forms FDA 3542a and 3542 Overview and Instructions?
1
Pharmaceutical companies submitting NDA applications.
2
Legal teams handling patent information.
3
Researchers needing to register patent details.
4
Regulatory compliance officers in drug companies.
5
Consultants assisting clients with FDA submissions.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the Forms FDA 3542a and 3542 Overview and Instructions along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your Forms FDA 3542a and 3542 Overview and Instructions online.
Editing this PDF on PrintFriendly is easy and intuitive. Simply upload your file and access the editing tools to make necessary changes. You can adjust text, insert new fields, and organize your document as needed.
Add your legally-binding signature.
Signing the PDF on PrintFriendly is a straightforward process. Once you've completed your document, use the e-signature feature to add your signature effortlessly. Save your signed document and share it as needed with confidence.
Share your form instantly.
Sharing your PDF on PrintFriendly is fast and efficient. After editing, use the sharing options to send your document via email or social media. You can also generate a shareable link for easier access.
How do I edit the Forms FDA 3542a and 3542 Overview and Instructions online?
Editing this PDF on PrintFriendly is easy and intuitive. Simply upload your file and access the editing tools to make necessary changes. You can adjust text, insert new fields, and organize your document as needed.
1
Upload your PDF to PrintFriendly.
2
Access the editing tools available.
3
Make the necessary changes to the document.
4
Review your edits for accuracy.
5
Download the edited PDF for your records.

What are the instructions for submitting this form?
To submit Form FDA 3542a, please send it via email to fda_nida@fda.gov, or fax it to (301) 827-1011. For online submissions, visit the FDA's electronic submission portal. Alternatively, you may mail the completed forms to the FDA Center for Drug Evaluation and Research, 10903 New Hampshire Avenue, Silver Spring, MD 20993, ensuring you keep a copy for your records.
What are the important dates for this form in 2024 and 2025?
Key dates for Forms FDA 3542a and 3542 include submission deadlines of 30 days post-NDA approval for Form FDA 3542. Patent information must be submitted and updated accordingly within specified time frames. Ensure you stay informed regarding any amendments in regulations for 2024 and 2025.

What is the purpose of this form?
Forms FDA 3542a and 3542 are essential for submitting patent information related to new drug applications. These forms facilitate compliance with FDA regulations by ensuring accurate and timely reporting of patent details. Understanding their purpose helps stakeholders navigate the patent submission process effectively.

Tell me about this form and its components and fields line-by-line.

- 1. Patent Information: Details regarding the patent associated with the NDA application.
- 2. NDA Application Number: The unique identifier for the New Drug Application.
- 3. Submission Date: The date when the form is officially submitted to the FDA.
- 4. Signature: The authorized signature verifying the information provided.
- 5. Contact Information: Details for reaching the submitter for follow-up questions.
What happens if I fail to submit this form?
Failing to submit Forms FDA 3542a and 3542 can lead to compliance issues with the FDA. This may result in delays in the approval of the NDA and potential legal repercussions. It's crucial to ensure timely and accurate submission of these forms.
- Approval Delays: Incomplete submissions can significantly prolong the review process.
- Legal Repercussions: Failure to comply with submission requirements may lead to legal action.
- Rejection of NDA: Inaccurate information may result in the outright rejection of the New Drug Application.
How do I know when to use this form?

- 1. Initial NDA Submission: Required during the first submission of a new drug patent.
- 2. Post-Approval Changes: Necessary for any updates to patent information after NDA approval.
- 3. Supplement Applications: Used when submitting amendments or supplements to existing NDAs.
Frequently Asked Questions
How can I edit Forms FDA 3542a and 3542?
You can easily edit these forms by uploading them to PrintFriendly and using our editing tools.
Is it possible to sign the FDA forms online?
Yes, you can sign the FDA forms right on PrintFriendly using our e-signature feature.
How do I share my completed FDA forms?
After editing, you can share your completed forms via email or social media easily.
Can I revert changes made to my PDF?
You can review your edits before downloading, allowing you to undo any changes as needed.
Are there any limitations when editing PDFs on PrintFriendly?
While you can edit and download, some features may be limited; however, we're continually improving.
What formats can I use for uploading documents?
PrintFriendly supports various document formats, primarily PDFs, for upload and editing.
How do I ensure my edits save correctly?
After making your edits, confirm your changes before downloading to ensure they are saved.
What if I encounter issues while editing?
Our support team is available to assist you with any issues you might face during editing.
Can I download the PDF after editing?
Yes, you can download the edited PDF directly from PrintFriendly.
Is there a limit to how many times I can edit a PDF?
You can edit and download your PDF as many times as you need.
Related Documents - FDA Forms 3542

FDA Recall Audit Check Report Instructions
This file provides detailed instructions for completing the FDA Recall Audit Check Report. It includes information on recall details, program data, audit accounts, and consignee data. Useful for those involved in managing FDA recalls.

Assessment of Abuse Potential of Drugs Guidance for Industry
This document provides guidance for the assessment of abuse potential in drugs. It covers key decision points, recommended studies, and the process for NDA submission. This is crucial for ensuring drug safety and regulatory compliance.

Nurtec ODT Savings Program Terms & Conditions
This document provides detailed terms and conditions for the Nurtec ODT Savings Program. It includes eligibility criteria, instructions for pharmacists, and important disclaimers. Patients using the copay card should adhere to these guidelines to benefit from the program.
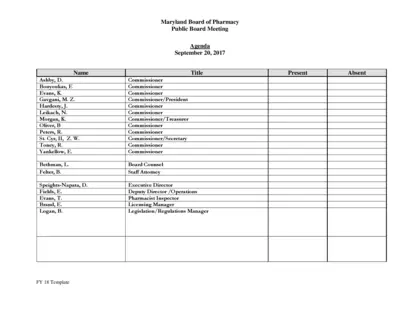
Maryland Board of Pharmacy Public Board Meeting Agenda
This file contains the agenda for the Maryland Board of Pharmacy's public board meeting on September 20, 2017. It includes reports from various committees and updates on operations, licensing, compliance, and more. The document is essential for stakeholders to keep track of board activities and decisions.

Abbreviations for Pharma Manufacturers
This file contains a list of manufacturers' abbreviations organized alphabetically, helping users to identify manufacturer names and their corresponding abbreviations.

Pharma-Lagom: Safe and Effective Medication Use
Pharma-Lagom is a comprehensive guide on the risks and benefits of medication use, aimed at promoting safe and effective medication practices. It includes contributions from experts in the Pharmacy Department of Kalaniketan Polytechnic College, Jabalpur. This document also covers recent events and achievements within the department.

MDUFMA User Fees Cover Sheet Instructions
The MDUFMA User Fees Cover Sheet is required for Medical Device Application Submission. It includes details on registration and payment processes. Follow this guide to complete and submit your form correctly.
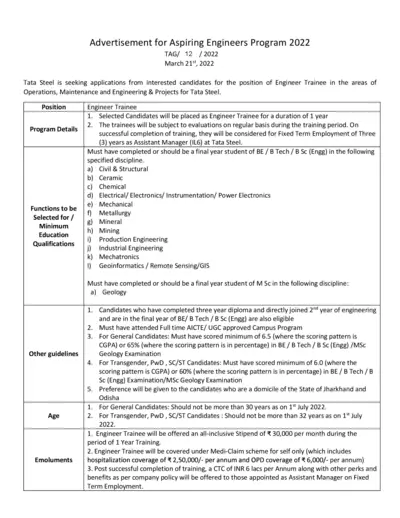
Tata Steel Aspiring Engineers Program 2022 Application
This file includes details about Tata Steel's Aspiring Engineers Program 2022. It covers program details, eligibility criteria, and the application process. It also provides information on evaluation, guidelines, and submission deadlines.

Welding Procedure Specification (WPS) PDF Guide
This file is a Welding Procedure Specification (WPS) that provides detailed instructions for welding procedures, joint design, base metals, filler metals, and more. It includes sections for prequalified and qualified-by-testing procedures. This document is essential for ensuring weld quality and consistency.

California Intern Pharmacist Application Instructions
This document provides detailed instructions for applying for an Intern Pharmacist license in California. It covers processing time, required materials, and special cases for expedited review. Ensure all requirements are met to avoid application delays.

Botox Cosmetic Patient Medication Information
This file contains detailed information about Botox Cosmetic (onabotulinumtoxinA). It includes dosage, administration, warnings, precautions, and adverse reactions. The document is intended for healthcare professionals and patients.

Join the Kings Club and Save Instantly with a Kings Club Card
Apply for a Kings Club Card at any of our locations and start saving instantly. Fill out the form in-store or online to receive your card. Enjoy discounts and additional benefits with your Kings Club membership.