Noble Gases Properties and Chemical Behavior
This document provides a comprehensive overview of the noble gases, their properties, and chemical behaviors. It includes information on individual gases, their electronic configurations, and compound formations. Ideal for students and professionals in chemistry.
Edit, Download, and Sign the Noble Gases Properties and Chemical Behavior
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out this document, start by reviewing the properties of noble gases outlined in the initial sections. Next, refer to the specific chemical properties and behaviors to understand their interactions. Finally, utilize the provided tables for detailed information.
How to fill out the Noble Gases Properties and Chemical Behavior?
1
Review the initial definitions and characteristics.
2
Understand the properties and behaviors of each gas.
3
Refer to the tables for specific data points.
4
Note the chemical interaction examples provided.
5
Use this information to complete your study or report.
Who needs the Noble Gases Properties and Chemical Behavior?
1
Chemistry students who require detailed information on noble gases.
2
Researchers focusing on gas properties in experimental studies.
3
Teachers looking for resources to explain noble gases.
4
Professionals in industries like lighting and welding using noble gases.
5
Environmental scientists studying the gaseous composition of the atmosphere.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the Noble Gases Properties and Chemical Behavior along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your Noble Gases Properties and Chemical Behavior online.
Editing this PDF on PrintFriendly is simple and user-friendly. Just open the document in our editor, where you can easily make your changes. Save your edits directly within the platform for a seamless experience.
Add your legally-binding signature.
You can sign the PDF using our PrintFriendly platform effortlessly. Simply access the signing tool and draw or upload your signature. Once completed, save your signed document.
Share your form instantly.
Sharing the PDF on PrintFriendly is straightforward. Use our sharing options to send the document via email or social media. It allows you to easily maintain document integrity while sharing.
How do I edit the Noble Gases Properties and Chemical Behavior online?
Editing this PDF on PrintFriendly is simple and user-friendly. Just open the document in our editor, where you can easily make your changes. Save your edits directly within the platform for a seamless experience.
1
Open the PDF in the PrintFriendly editor.
2
Select text or images you wish to edit.
3
Make your changes using the editing tools provided.
4
Review your edits for accuracy.
5
Save the updated document to your device.

What are the instructions for submitting this form?
To submit this form, ensure you have filled out all required fields correctly. If you are submitting via email, send it to submissions@example.com including subject line 'Noble Gases Submission.' For physical submission, mail to the address: 123 Science Lane, Chemistry Building, City, State, Zip.
What are the important dates for this form in 2024 and 2025?
No specific important dates are applicable for this document.

What is the purpose of this form?
The purpose of this form is to provide a concise and informative overview of noble gases that can be used for educational and professional reference. It is designed to assist both students and industry professionals in understanding the unique characteristics and applications of noble gases. This comprehensive resource will enhance knowledge and provide a solid foundation in the study of these crucial elements.

Tell me about this form and its components and fields line-by-line.

- 1. Element: Name of the noble gas element.
- 2. Electronic Configuration: The electronic arrangement of the atom.
- 3. Boiling Point (K): The boiling point of the gas in Kelvin.
- 4. Melting Point (K): The melting point of the gas in Kelvin.
- 5. Density: Density of the gas at boiling point in g/m3.
- 6. Ionization Energy: The amount of energy required to remove an electron.
What happens if I fail to submit this form?
Failure to submit this form results in the inability to access the critical information regarding noble gases. You may miss essential data for educational or professional use.
- Missing Information: Users will lack access to valuable properties and chemical behavior of noble gases.
- Inability to Complete Tasks: Educational or research tasks may be incompletely performed.
- Loss of Time: Time may be wasted trying to compile this information independently.
How do I know when to use this form?

- 1. Academic Research: Ideal for students and researchers looking for comprehensive data on noble gases.
- 2. Educational Purposes: Teachers and educators can use this for lesson planning.
- 3. Industrial Applications: Professionals in industries that utilize noble gases can refer to this document.
Frequently Asked Questions
How can I edit this PDF?
You can edit the PDF by opening it in the PrintFriendly editor and using the tools available.
What formats can I export this document?
You can export this document as a PDF file.
Can I sign this PDF digitally?
Yes, you can add your signature using the signature tools provided.
Is there an option to share the PDF?
Absolutely! You can share the PDF directly through email or social media.
What should I do if I have issues editing?
Ensure you are using a compatible browser and try refreshing the page.
Can I download the edited file?
Yes, after editing you can download the updated PDF directly.
Will my changes be saved automatically?
Yes, you need to click save after editing to ensure your changes are stored.
Is there a limit to how much I can edit?
There are no restrictions on the amount of content you can edit in the PDF.
Can I undo changes?
Yes, there is an undo option to revert your last edits.
Is assistance available if I face technical issues?
Help is available through our support channels for any technical difficulties.
Related Documents - NobleGasesOverview

Understanding Form and Form-Perception by James J. Gibson
This document explores various definitions and theories of form, emphasizing the need for precise terminology. It delves into experiments related to the visual perception of form, distinguishing between solid and surface forms. The text critiques traditional views and presents new perspectives on form-perception.

Prehistory of Peer Review: Religious Blueprints
This file explores the origins and development of peer review in science, tracing its roots to religious scholars in the Hartlib circle. It discusses the influence of the Royal Society of London and other early scientific organizations. The content is based on extensive historical research and analysis.

Analysis of Uncertainties in Radioactivity Measurements
This document discusses the uncertainties in analytical radioactivity measurements of environmental samples. It includes detailed equations and methods for calculating total propagated uncertainties. Useful for quality assurance specialists, data validators, and radiochemical analysts.

IRMS Sample Analysis Request Form Guidelines
This file contains instructions and details about the IRMS Sample Analysis Request Form. It is used to request sample analysis in the Laboratory for Isotopes and Metals in the Environment. Ensure you have the required approvals before using the IRMS.

Double Stuff Oreo Cookie Science Experiment
This file contains details and instructions for conducting a science experiment to evaluate the marketing claim of Double-Stuff Oreo cookies. Users will measure the mass of regular and Double-Stuff Oreo cookies along with their fillings. It guides users through the process of data collection, calculation, and analysis using the scientific method.
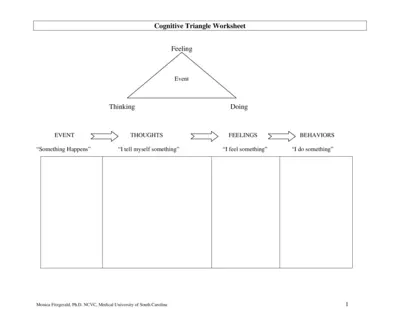
Cognitive Triangle Worksheet Instructions and Details
This file provides an overview and detailed instructions on how to use the Cognitive Triangle Worksheet. It helps users understand the relationship between their thoughts, feelings, and behaviors. Perfect for those interested in cognitive-behavioral strategies.

Engaging Doctor Pretend Play Printables for Kids
Transform playtime with free doctor pretend play printables designed for kids. These fun tools foster creativity and learning through imaginative play. Perfect for children from toddlers to first graders.

Buffer Solutions: Understanding Their Functionality
This file provides comprehensive insights into buffer solutions, including their preparation and pH resistance mechanisms. Ideal for chemistry students and professionals seeking to understand buffer systems. Practice problems included for hands-on learning.

Ions and Ionic Compounds: Understanding Nomenclature
This file provides a comprehensive overview of ions, including their types, charges, and nomenclature rules. It covers essential details such as simple and polyatomic ions, and how to name them correctly. Perfect for students and professionals looking to deepen their understanding of ionic compounds.

Biology Form 3 Notes and Instructions
This file contains detailed biology notes for Form 3 students. It covers essential topics such as organism classification and characteristics of various kingdoms. Perfect for studying and exam preparation.

Chemical Bonding and Matter in Our Surroundings
This file provides detailed insights into chemical bonding, including ionic and covalent bonds. It covers the principles of matter in our surroundings and the electronic configurations of elements. Ideal for students and educators in chemistry to enhance their understanding.
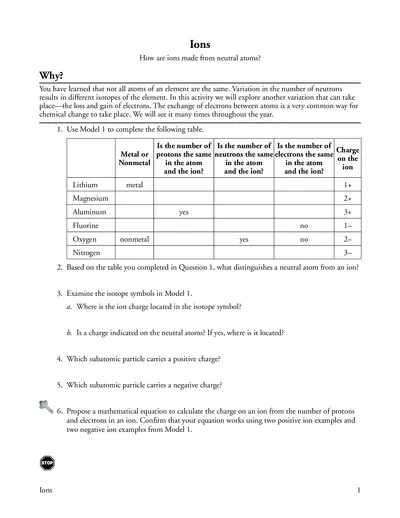
Understanding Ions and Their Formation in Chemistry
This file provides comprehensive insights into the formation of ions from neutral atoms. It covers the characteristics of cations and anions and explores the chemical changes involved. Perfect for students and educators seeking to understand atomic variations.