SMART IRB Audit Checklist for Regulatory Compliance
This file contains a comprehensive audit checklist for ensuring compliance with regulatory requirements in clinical research. Designed for use by auditors and research staff, it provides step-by-step guidance on necessary documentation and procedural adherence. Utilize this audit checklist to ensure all aspects of IRB-approved protocols are met efficiently.
Edit, Download, and Sign the SMART IRB Audit Checklist for Regulatory Compliance
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill out this checklist, start by gathering all relevant regulatory documents associated with the study. Review each question carefully and provide accurate responses based on existing documentation. Ensure that all necessary signatures and approvals are in place before submission.
How to fill out the SMART IRB Audit Checklist for Regulatory Compliance?
1
Collect all required regulatory documentation.
2
Carefully review each item on the checklist.
3
Provide accurate and complete responses.
4
Ensure necessary approvals and signatures are obtained.
5
Submit the completed checklist for review.
Who needs the SMART IRB Audit Checklist for Regulatory Compliance?
1
Clinical research coordinators need this file for ensuring compliance during audits.
2
Principal Investigators utilize the checklist to verify all necessary documents are in place.
3
IRB members refer to the checklist to assess study compliance effectively.
4
Auditors use this checklist to evaluate adherence to regulatory standards.
5
Regulatory affairs specialists need it for preparing submissions to oversight bodies.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the SMART IRB Audit Checklist for Regulatory Compliance along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your SMART IRB Audit Checklist for Regulatory Compliance online.
On PrintFriendly, editing this PDF is simple and efficient. You can easily make necessary changes and annotations directly on the document. Our intuitive interface allows for seamless modifications to meet your needs.
Add your legally-binding signature.
Signing this PDF on PrintFriendly is a straightforward process. You can add your signature electronically, ensuring a legally-binding consent. The user-friendly tools make it easy to place your signature wherever needed.
Share your form instantly.
Sharing this PDF document is effortless on PrintFriendly. You can quickly send the file via email or share it through your preferred social platforms. Ensure all relevant parties have access to the important information contained within.
How do I edit the SMART IRB Audit Checklist for Regulatory Compliance online?
On PrintFriendly, editing this PDF is simple and efficient. You can easily make necessary changes and annotations directly on the document. Our intuitive interface allows for seamless modifications to meet your needs.
1
Upload the PDF document you want to edit.
2
Use the available editing tools to make changes.
3
Add comments or annotations as needed.
4
Save your edits when you're finished.
5
Download or share your edited document.

What are the instructions for submitting this form?
To submit the completed checklist, send it via email to the designated IRB office at irb@institution.edu. Alternatively, you can fax the document to (123) 456-7890. Physical submissions can be mailed to the IRB office at 123 Research Rd, Suite 456, City, State, ZIP. Always ensure that submission deadlines are met and confirm receipt of your documents.
What are the important dates for this form in 2024 and 2025?
Important dates for the compliance review process typically include the initial submission deadline for protocols, annual review dates set by the IRB, and any required audit dates specified by regulatory agencies. Please check specific timelines within your institution or associated regulatory body for 2024 and 2025.

What is the purpose of this form?
The purpose of this form is to ensure that all regulatory documentation required for clinical research audits is adequately reviewed and maintained. It serves as a checklist for compliance with Institutional Review Board (IRB) protocols and federal regulations. By systematically confirming each aspect of the audit requirements, researchers and auditors can uphold the integrity of clinical studies.

Tell me about this form and its components and fields line-by-line.

- 1. Approved Protocol: Verification of the presence of the approved study protocol.
- 2. IRB Approval Letter: Confirmation of the IRB Approval Letter's availability.
- 3. FDA Regulated Study: Indication of whether the study falls under FDA jurisdiction.
- 4. Staff Training Log: Documentation of staff training and competency.
- 5. Subject Enrollment Log: Record of participant enrollment and demographics.
What happens if I fail to submit this form?
Failing to submit this checklist can lead to regulatory non-compliance. This may prompt audits or delays in the research process. It's crucial to complete and submit all necessary documentation on time.
- Regulatory Delays: Missing submissions can cause delays in regulatory approvals.
- Audit Non-Compliance: Inadequate documentation may result in audit findings against the organization.
- Inability to Proceed: Research activities may be temporarily halted due to incomplete submissions.
How do I know when to use this form?

- 1. Audit Preparation: Utilize this checklist to prepare for upcoming audits.
- 2. IRB Applications: Refer to this checklist when submitting new applications or amendments.
- 3. Regulatory Reviews: Use during routine regulatory reviews to ensure compliance.
Frequently Asked Questions
How do I access the SMART IRB audit checklist?
You can download the checklist directly from our print-friendly page to get started.
Can I edit the checklist PDF?
Yes, you can edit the checklist on PrintFriendly using our PDF editing tools.
Is it possible to share the checklist with others?
Absolutely, you can easily share the checklist via email or social media.
What should I do if I need to sign the checklist?
You can add your signature electronically using our signing feature on PrintFriendly.
How can I ensure my checklist is compliant?
Follow the provided guidelines and review each section thoroughly to ensure compliance.
What if I make a mistake while filling out the checklist?
You can easily go back and edit any section of the checklist as needed.
Do I need specific software to use the checklist?
No, all functionalities are available directly on the PrintFriendly website.
Can I print the checklist directly from the site?
Yes, you can print the checklist from PrintFriendly after editing.
What happens if I submit an incomplete checklist?
It may delay the approval process, so ensure completeness before submission.
Is there a deadline for submitting the checklist?
Check with your IRB or regulatory body for specific submission deadlines.
Related Documents - SMART IRB Audit Checklist

Nissan Graduate & Placement Schemes: Opportunities & Application Process
This file provides details about Nissan's graduate and placement schemes, including hands-on experience and multi-million pound projects. It covers life at Nissan Sunderland, scheme types, and the application process. Ideal for ambitious graduates seeking a career in the automotive industry.

Nebraska Electronic Lien and Title Change Request Form
This form is used by vehicle owners and lenders to request changes to Nebraska electronic titles, such as name change, adding or removing an owner, or updating Transfer-on-Death designation.

CPS Guarantee of Title and Buyer Information Form
This file is the CPS Guarantee of Title form, which includes sections for buyer information, vehicle details, lien recording information, and dealership authorization. The form guarantees payment to Consumer Portfolio Services, Inc. and remains in effect until the title certificate is delivered. It's required for the proper recording and authorization of vehicle purchase agreements.

Engine Build Sheets and Blueprint Records
This file contains detailed engine build sheets and blueprint records for Mopar big-block engines. It provides specifications, measurements, and part numbers required for engine assembly. Essential for accurate engine building and performance tuning.

General Motors Product Field Action Reimbursement Form
This document is a reimbursement request form for repairs covered under General Motors Product Field Action. It guides customers on how to fill out and submit the form for reimbursement. Includes sections for both customer and dealer to complete.

Vehicle Release of Liability Form
A document to formalize the sale and transfer of ownership of a vehicle from a seller to a buyer. It includes terms for vehicle sale, payment, and liability. Essential for legal protection during vehicle transactions.

Instructions for Transferring a Texas Vehicle Title
This document provides detailed instructions on how to transfer a vehicle title in Texas. It outlines the requirements for both the seller and the buyer. The form must be completed and submitted within 30 days to avoid penalties.
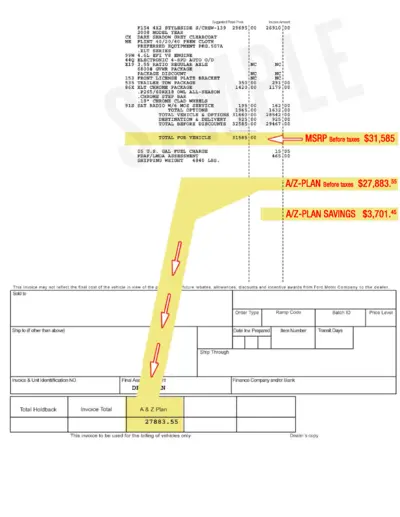
2008 Ford F154 Styleside Invoice Details
This file includes the detailed invoice of a 2008 Ford F154 Styleside. It lists out the suggested retail price, various equipment packages, and total costs. Use this file to understand the cost breakdown for the vehicle.

General Motors Accessibility Reimbursement Application Form
This file contains the General Motors Accessibility Reimbursement Application form. It provides instructions on how to apply for reimbursement for adaptive equipment installed in GM vehicles. Follow the steps to complete your application and submit it for approval.

Kansas Department of Revenue - Bill of Sale Form
This form is used by the Kansas Department of Revenue to document the sale or transfer of ownership of a vehicle. It is specifically for antique vehicles 35 years old or older. The form needs to be filled out by both the seller and buyer.

TURO Required Maintenance Inspection Checklist
This document is a comprehensive maintenance inspection checklist required for TURO hosts. It covers various inspection points ranging from brakes to batteries. The checklist ensures that the vehicle meets the safety standards set by TURO.

Ford/Lincoln Extended Service Plan Transfer Waiver
This document is used to transfer the Ford/Lincoln Extended Service Plan from the current owner to a new owner. It includes the necessary fields to capture information about both parties and the vehicle. By filling out this form, the original owner relinquishes their rights to cancel the contract and receive any refunds.