Environmental Protection Documents

Environmental Advocacy
Human Rights Education Toolkit Instructions Manual
This document serves as a comprehensive toolkit for writing effective advocacy letters regarding human rights issues. It includes guidelines on how to structure your letter and details on the necessary components to make your request impactful. Ideal for individuals and organizations seeking to influence change in their communities.

Renewable Energy
TANESCO 2017 Tariff Adjustment Application
This document outlines Tanzania Electric Supply Company's proposed tariff adjustments for 2017. It includes regulatory applications, current and proposed tariffs, and customer benefits. Use this file for understanding TANESCO’s tariff proposals and the regulatory framework surrounding it.

Regulatory Permits
JPMorgan Comments on Regulation E Proposed Rule 2009
This document contains JPMorgan Chase's comments regarding the proposed amendments to Regulation E. It addresses concerns about service fees related to prepaid cards. The comments aim to clarify definitions and provide suggestions for better consumer understanding.

Regulatory Permits
FDA 510(k) Submission for neuromate Gen III Device
This document details the FDA's premarket notification decision for the neuromate Gen III medical device. It provides insights into compliance and regulatory requirements. This file is essential for manufacturers and healthcare professionals involved in device approval.

Regulatory Permits
REACH SVHC Declaration Form - CommScope Compliance
This file contains the REACH SVHC Declaration Form from CommScope, detailing product compliance. It provides essential information on substances in articles according to ECHA guidelines. Users can understand product safety and compliance through this document.

Renewable Energy
APR Energy Solutions for Temporary Power Generation
This file provides an overview of APR Energy's flexible power generation solutions and technologies. Users can learn about temporary power services offered by APR Energy in various applications, including disaster recovery. It's an informative resource for businesses and organizations needing reliable energy solutions.

Environmental Advocacy
Parent Toolkit for Student Privacy Petition
This file provides a sample petition for parents advocating for better privacy protections for students. It includes key principles for protecting student data. This resource is essential for engaging the school community in discussions about student privacy.

Wildlife Protection
Application for Zoo Recognition Under Wildlife Act
This file is an application form to seek recognition from the Central Zoo Authority under the Wildlife Protection Act. It includes required details about the zoo such as its establishment date, location, animal stock, and facilities. Completing this form is crucial for zoos seeking official recognition.
Renewable Energy
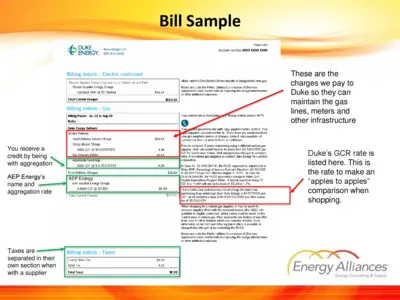
Duke Energy Billing Instructions and Details
This document provides comprehensive billing details and instructions for Duke Energy customers. It includes information on electric and gas charges, taxes, and supplier options. Users can utilize this file to better understand their billing statement and make informed choices regarding energy suppliers.

Regulatory Permits
CFTC Form 304 Cash Positions Reporting Guide
The CFTC Form 304 is essential for reporting unfixed-price Cotton 'On Call' positions. This form helps traders comply with Commodity Exchange Act requirements. Understanding and submitting this form accurately ensures compliance and avoids penalties.
Renewable Energy
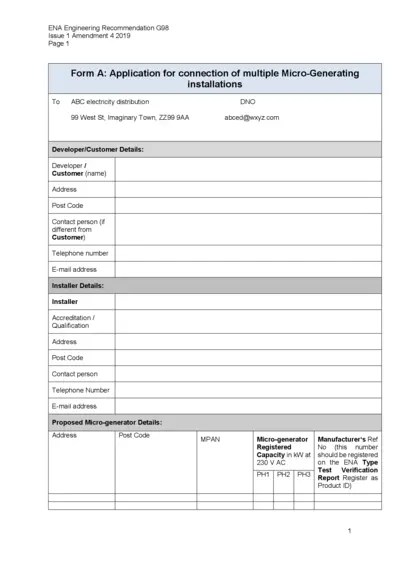
ENA Engineering Recommendation G98 Application Form
This is the ENA Engineering Recommendation G98 Application form for connection of Micro-Generating installations. It provides detailed instructions and requirements for developers and installers. Use this form to submit your application to the electricity distribution network operator.
Regulatory Permits
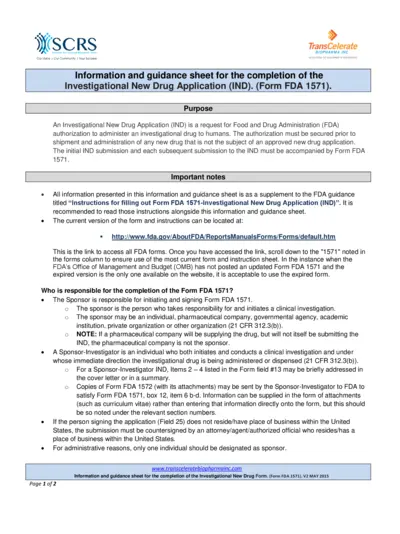
Investigational New Drug Application Submission Guide
This document serves as a comprehensive guide for completing the Investigational New Drug Application (IND), specifically Form FDA 1571. It provides essential information, guidance, and tips for sponsors and investigators, ensuring compliance with FDA regulations. Users will find detailed instructions and important notes to streamline their application process.