Drug Development Documents

Clinical Trials
Transfusion Biotin-Labeled Red Blood Cells Study
This clinical protocol outlines the study involving the transfusion of biotin-labeled red blood cells. It focuses on evaluating genetic factors contributing to donor differences. The file serves as a comprehensive guide for researchers and healthcare professionals involved in the study.
Clinical Trials
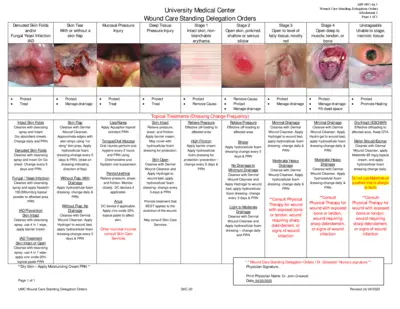
Wound Care Standing Delegation Orders Overview
This file provides comprehensive instructions and protocols for wound care management following standing delegation orders. It includes information about treatment for various skin conditions such as skin tears, fungal infections, and pressure injuries. Designed for healthcare professionals, this document serves as an essential guide for effective wound care.

FDA Regulations
Form FDA 3674 Certifications for Drug Applications
The FDA 3674 form provides essential certifications required for submitting drug, biological product, and device applications. This guidance helps sponsors, researchers, and investigations meet compliance standards. It's crucial for ensuring that necessary certifications accompany relevant applications.
Clinical Trials
Weekly Activity Schedule for Clinical Interventions
This file provides a structured weekly activity schedule for planning. It is ideal for clinical interventions and training programs. Refer to this document to effectively organize daily activities.

Drug Testing
Drug Screen Point of Care Test Result Form
This document provides a comprehensive guide for drug testing at the point of care. It includes donor information, collector verification, and result interpretation. Suitable for use in various testing scenarios including pre-employment and random testing.

Generic Drugs
D-Lysergic Acid Diethylamide Drug Overview
This document provides comprehensive details about D-Lysergic Acid Diethylamide (LSD), including its uses, effects, and user statistics. It is crucial for anyone looking to understand this potent hallucinogen. The file also covers its illicit uses and clinical observations.
Clinical Trials
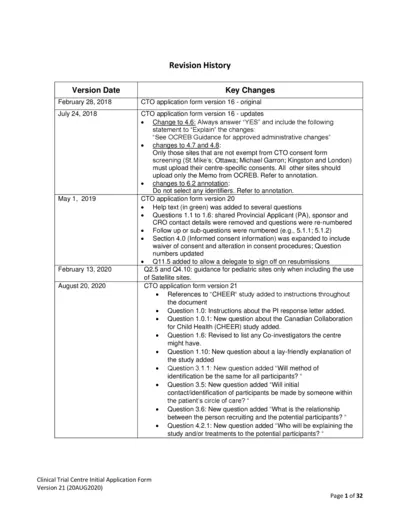
CTO Clinical Trial Centre Initial Application Form
This document provides essential information and guidelines for submitting the Clinical Trial Centre Initial Application Form for research ethics approvals. It outlines revisions, updates, and crucial components for successfully filling out the form. Researchers and coordinators will find valuable instructions on compliance and submission processes.

Generic Drugs
Revised Labeling Review for Orfadin Documentation
This file contains a review memorandum for the revised label and labeling of Orfadin (nitisinone). It details the evaluation conducted by DMEPA regarding medication error prevention. This document is essential for understanding the labeling requirements and compliance standards.

FDA Regulations
FDA PMA Postapproval Requirements Guide
This document provides comprehensive guidelines for the FDA's Premarket Approval Application (PMA) program and relevant postapproval requirements. Users will find instructions about submitting reports, amendments, and periodic updates. Essential for regulatory compliance and understanding the responsibilities post-approval.

FDA Regulations
FDA Export Certificate Request Guidelines
This file provides important guidelines for submitting export certificate requests to the FDA. It outlines the necessary information and the process for obtaining certificates for various products. Ideal for manufacturers and distributors looking for compliance with FDA regulations.

FDA Regulations
FDA Written Request for Pediatric Studies for Sofosbuvir
This file contains the FDA's written request regarding pediatric studies for sofosbuvir. It outlines the requirements for study submissions. The document also includes essential information and guidance for the involved parties.
Clinical Trials
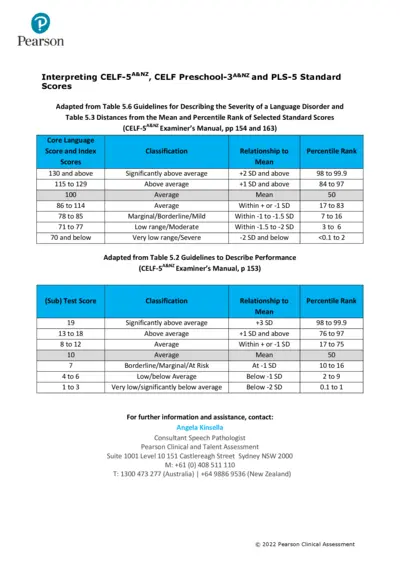
CELF-5A&NZ Language Disorder Assessment Guide
This document provides detailed guidelines for interpreting the CELF-5A&NZ and related assessments. It outlines severity classifications and scoring criteria for evaluating language disorders. A must-read for clinicians and educators involved in language assessment.