Understanding Chemical Bonding and Compounds
This file provides an insightful overview of chemical bonding in both ionic and molecular compounds. It compares the properties of ionic and molecular compounds and explains how they differ in behavior and characteristics. Ideal for chemistry students and educators seeking to deepen their understanding of chemical formulas and bonding principles.
Edit, Download, and Sign the Understanding Chemical Bonding and Compounds
Form
eSign
Add Annotation
Share Form
How do I fill this out?
To fill this file out, start by reviewing the chemical formulas provided. Next, compare the properties and characteristics of ionic and molecular compounds. Finally, follow the provided examples of Lewis structures and crystal lattice formations to complete your understanding.
How to fill out the Understanding Chemical Bonding and Compounds?
1
Review the provided chemical formulas.
2
Identify whether the compounds are ionic or molecular.
3
Compare the properties of each compound.
4
Understand the examples of Lewis structures.
5
Complete the information by following the guidelines.
Who needs the Understanding Chemical Bonding and Compounds?
1
Chemistry students needing to grasp bonding concepts.
2
Educators looking for materials to teach chemical bonding.
3
Researchers seeking foundational knowledge in chemistry.
4
Academics preparing for advanced chemistry studies.
5
Professionals in related fields needing a reference on compounds.
How PrintFriendly Works
At PrintFriendly.com, you can edit, sign, share, and download the Understanding Chemical Bonding and Compounds along with hundreds of thousands of other documents. Our platform helps you seamlessly edit PDFs and other documents online. You can edit our large library of pre-existing files and upload your own documents. Managing PDFs has never been easier.
Edit your Understanding Chemical Bonding and Compounds online.
Editing this PDF on PrintFriendly is simple and user-friendly. You can click on the text you want to modify and make your changes directly. After editing, be sure to save your updated version to keep your revisions.
Add your legally-binding signature.
You can sign the PDF on PrintFriendly by entering your signature in the designated area. Once you've entered your signature, it will be embedded in the document. This feature allows for easy, digital authorization without needing to print the file.
Share your form instantly.
Sharing the PDF on PrintFriendly is a breeze. After completing your edits, you can generate a shareable link to your document. This makes collaboration effortless and efficient for your team.
How do I edit the Understanding Chemical Bonding and Compounds online?
Editing this PDF on PrintFriendly is simple and user-friendly. You can click on the text you want to modify and make your changes directly. After editing, be sure to save your updated version to keep your revisions.
1
Open the PDF in PrintFriendly.
2
Click on the text you wish to edit.
3
Make the necessary changes directly in the document.
4
Review your edits for accuracy.
5
Save your changes to keep your updated PDF.

What are the instructions for submitting this form?
To submit the form, ensure all sections are filled out completely. You can send it via email to your instructor or submit it through the online learning portal. Alternatively, you may choose to print the form and provide a physical copy to your educational institution, ensuring all necessary details are included.
What are the important dates for this form in 2024 and 2025?
Important dates related to this document will vary by academic institution. Typically, it is relevant for the duration of the chemistry course being taught. Refer to your course syllabus for specific dates.

What is the purpose of this form?
The purpose of this form is to provide clear guidance and instruction on understanding chemical bonding principles. It delves into the differences between ionic and molecular compounds, offering practical examples for learners. Moreover, it serves as a foundational document for both theoretical and practical applications in chemistry education.

Tell me about this form and its components and fields line-by-line.

- 1. Chemical Formulas: Shows the structure of compounds.
- 2. Comparison of Compounds: Highlights differences between ionic and molecular bonds.
- 3. Lewis Structures: Illustrates the arrangement of atoms in molecules.
- 4. Crystal Lattice Models: Demonstrates the structure of ionic compounds.
What happens if I fail to submit this form?
If you fail to submit this form, you may miss critical insights into chemical bonding. Important concepts may not be effectively learned or applied. Ensuring timely submission is vital for academic success.
- Incomplete Understanding: Lack of submission can lead to gaps in knowledge.
- Missed Opportunities: You might miss out on vital educational resources.
- Delays in Learning: Failure to submit could hinder progress in your studies.
How do I know when to use this form?

- 1. Studying Chemistry: Ideal for students seeking to deepen their understanding.
- 2. Teaching Tool: Useful for educators conducting lessons on chemical bonding.
- 3. Reference for Research: Helps researchers quickly access bonding information.
Frequently Asked Questions
What type of content does this file cover?
This file covers essential concepts of chemical bonding, including ionic and molecular compounds.
Can I edit this PDF file?
Yes, you can edit this PDF directly in PrintFriendly without hassle.
How can I download the edited PDF?
After making edits, simply click the download button to save your changes.
Is this content suitable for beginners?
Absolutely! This file is designed to assist both beginners and advanced learners.
Are there examples included in the PDF?
Yes, the file includes various examples and illustrations of chemical structures.
Can I share this document with others?
Yes, PrintFriendly allows you to generate a shareable link for easy collaboration.
What if I need to print the document?
You can print the PDF directly after editing it to your satisfaction.
Does this file include instructions on how to fill it out?
Yes, the file provides clear instructions on completing its sections.
Who can benefit from this file?
Students, educators, and professionals in chemistry fields can all benefit from this document.
Is there a way to sign the PDF digitally?
Yes, you can sign the PDF directly on PrintFriendly.
Related Documents - ChemBondingGuide

Understanding Form and Form-Perception by James J. Gibson
This document explores various definitions and theories of form, emphasizing the need for precise terminology. It delves into experiments related to the visual perception of form, distinguishing between solid and surface forms. The text critiques traditional views and presents new perspectives on form-perception.

Prehistory of Peer Review: Religious Blueprints
This file explores the origins and development of peer review in science, tracing its roots to religious scholars in the Hartlib circle. It discusses the influence of the Royal Society of London and other early scientific organizations. The content is based on extensive historical research and analysis.

Analysis of Uncertainties in Radioactivity Measurements
This document discusses the uncertainties in analytical radioactivity measurements of environmental samples. It includes detailed equations and methods for calculating total propagated uncertainties. Useful for quality assurance specialists, data validators, and radiochemical analysts.

IRMS Sample Analysis Request Form Guidelines
This file contains instructions and details about the IRMS Sample Analysis Request Form. It is used to request sample analysis in the Laboratory for Isotopes and Metals in the Environment. Ensure you have the required approvals before using the IRMS.

Double Stuff Oreo Cookie Science Experiment
This file contains details and instructions for conducting a science experiment to evaluate the marketing claim of Double-Stuff Oreo cookies. Users will measure the mass of regular and Double-Stuff Oreo cookies along with their fillings. It guides users through the process of data collection, calculation, and analysis using the scientific method.
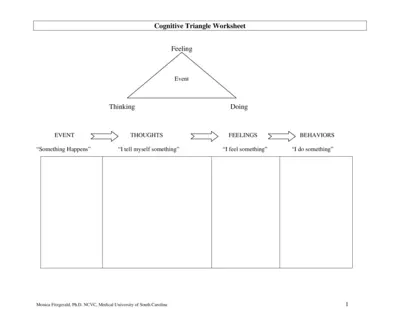
Cognitive Triangle Worksheet Instructions and Details
This file provides an overview and detailed instructions on how to use the Cognitive Triangle Worksheet. It helps users understand the relationship between their thoughts, feelings, and behaviors. Perfect for those interested in cognitive-behavioral strategies.

Engaging Doctor Pretend Play Printables for Kids
Transform playtime with free doctor pretend play printables designed for kids. These fun tools foster creativity and learning through imaginative play. Perfect for children from toddlers to first graders.

Buffer Solutions: Understanding Their Functionality
This file provides comprehensive insights into buffer solutions, including their preparation and pH resistance mechanisms. Ideal for chemistry students and professionals seeking to understand buffer systems. Practice problems included for hands-on learning.

Ions and Ionic Compounds: Understanding Nomenclature
This file provides a comprehensive overview of ions, including their types, charges, and nomenclature rules. It covers essential details such as simple and polyatomic ions, and how to name them correctly. Perfect for students and professionals looking to deepen their understanding of ionic compounds.

Biology Form 3 Notes and Instructions
This file contains detailed biology notes for Form 3 students. It covers essential topics such as organism classification and characteristics of various kingdoms. Perfect for studying and exam preparation.
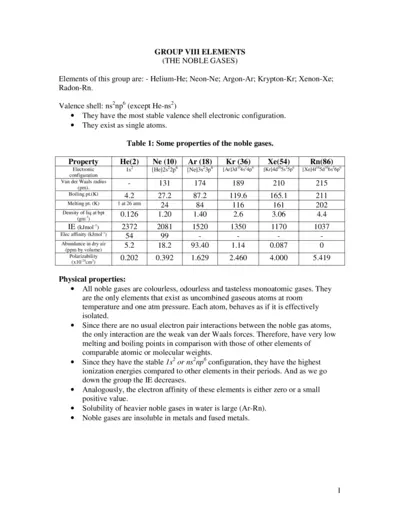
Noble Gases Properties and Chemical Behavior
This document provides a comprehensive overview of the noble gases, their properties, and chemical behaviors. It includes information on individual gases, their electronic configurations, and compound formations. Ideal for students and professionals in chemistry.

Chemical Bonding and Matter in Our Surroundings
This file provides detailed insights into chemical bonding, including ionic and covalent bonds. It covers the principles of matter in our surroundings and the electronic configurations of elements. Ideal for students and educators in chemistry to enhance their understanding.