Pharmaceuticals Documents

Synthetic Biology
Evolutionary History of Marine Mammals and Ecology
This file explores the phylogenetic history of marine mammals, detailing the evolution of species like seals, whales, and dugongs. It examines the transition from terrestrial to aquatic lifestyles and discusses various aspects of evolutionary biology relevant to marine mammals. Ideal for students, researchers, and enthusiasts of marine biology and evolution.
Clinical Trials
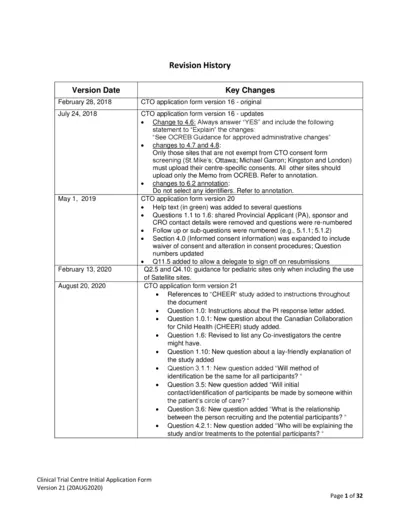
CTO Clinical Trial Centre Initial Application Form
This document provides essential information and guidelines for submitting the Clinical Trial Centre Initial Application Form for research ethics approvals. It outlines revisions, updates, and crucial components for successfully filling out the form. Researchers and coordinators will find valuable instructions on compliance and submission processes.
Genetic Engineering
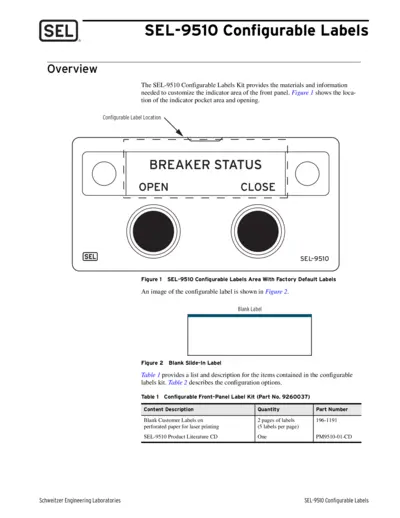
SEL-9510 Configurable Labels Overview and Instructions
The SEL-9510 Configurable Labels Kit provides users with the materials needed to customize the indicator areas of the SEL-9510 front panel. This guide includes options for existing labels and instructions for creating personalized label solutions. Explore the configuration options to tailor your labeling needs effectively.

Pharmaceutical Donations
MDA Donation Form for Strength and Independence
This document is a donation form for the Muscular Dystrophy Association. It outlines donation options and provides instructions for completing the form. Ideal for those wishing to support MDA and its initiatives.

Generic Drugs
Revised Labeling Review for Orfadin Documentation
This file contains a review memorandum for the revised label and labeling of Orfadin (nitisinone). It details the evaluation conducted by DMEPA regarding medication error prevention. This document is essential for understanding the labeling requirements and compliance standards.

FDA Regulations
FDA PMA Postapproval Requirements Guide
This document provides comprehensive guidelines for the FDA's Premarket Approval Application (PMA) program and relevant postapproval requirements. Users will find instructions about submitting reports, amendments, and periodic updates. Essential for regulatory compliance and understanding the responsibilities post-approval.

Pharmaceutical Donations
CHS Gift In-Kind Donation Submission Form
This form allows donors and CHS team members to submit GIK donation information for tax receipts and funding requirements. It guides users through entering details about items, services, and facilities donated. By using this form, users help CHS secure essential match funding and grant opportunities.
Genetic Engineering
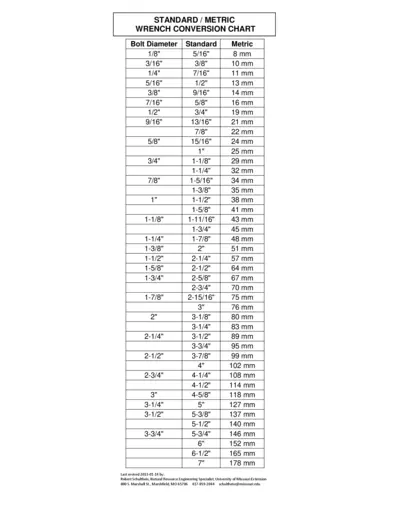
Wrench Conversion Chart Standard and Metric
This file contains a comprehensive wrench conversion chart that compares standard and metric bolt dimensions. It serves as a quick reference for mechanics and engineers to easily convert between different measurement systems. Ideal for anyone involved in construction, vehicle repair, or engineering.
Pharmaceutical Donations
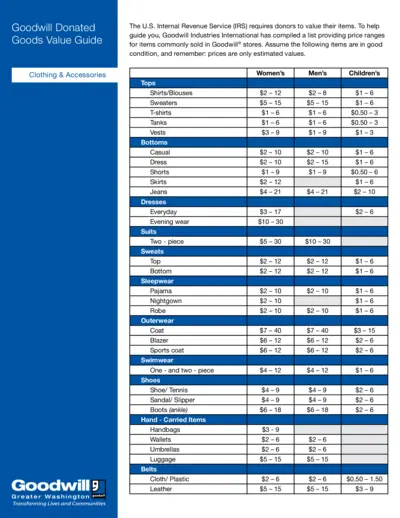
Goodwill Donated Goods Value Guide for IRS Compliance
This file provides a comprehensive value guide for donated goods to Goodwill. It helps donors estimate the value of their items for IRS reporting. Use it to determine fair market values for items typically sold in Goodwill stores.

Genetic Engineering
Clinton Iowa First Avenue Sewer Repair Documents
This file contains the bid documents for the First Avenue Sewer Repair project in Clinton, Iowa. It includes rules and deadlines for bid submission along with engineering certifications. This resource is essential for anyone involved in the bidding or approval processes.

Genetic Engineering
Zero Voltage Switching Resonant Power Conversion
This file explores the technique of zero voltage switching in modern power conversion. It discusses ZVS topologies, applications, and design procedures, along with example converters. The advantages of ZVS converters compared to traditional square wave counterparts are also analyzed.

Genetic Engineering
ASEAN Engineering Registry Application Guidelines
This document provides comprehensive guidelines and qualification requirements for the ASEAN Engineering Registry. It outlines how engineers can apply for registration and the necessary steps to complete the application process. The guidelines are essential for engineers seeking recognition and certification in the ASEAN region.